What is Hydroquinone?
Topical hydroquinone (HQ) is the gold standard and most commonly used clinical treatment for hyperpigmentation, freckles, sunspots, and melasma. Topical treatments for pigmentation generally focus on inhibiting tyrosinase, the enzyme that is critical for melanin synthesis to reduce pigment production. HQ is a phenolic compound (chemical name: 1,4-dihydroxybenzene) that works in several different ways to inhibit tyrosinase. It can also selectively damage melanocytes to reduce their function.
HQ is frequently combined with a prescription retinoid to enhance its penetration into the skin, thus increasing efficacy. Because it can cause irritation, redness and stinging, hydroquinone and prescription retinoids are frequently combined into a triple treatment with a steroid to reduce side effects.
It is available by prescription-only in most countries, although concentrations up to 2% can be obtained over the counter in some countries such as Australia.
Myth busting – exogenous ochronosis
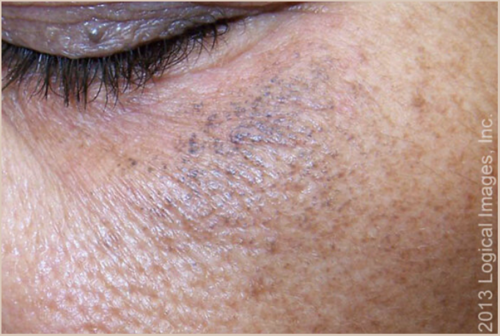
Even though HQ is such an effective and commonly used treatment, it suffers from bad PR (thanks, internet). The #1 thing that worries people is something with the terrifying-sounding name of exogenous ochronosis (EO)⠀
EO is a blue-grey skin discolouration on sun-exposed areas of the face, neck & upper chest/back. It is also a great example of how misinformation spreads disproportionately far and wide on the internet and social media compared to the truth.⠀
Let’s put things into perspective about how rarely EO is actually reported, and under what circumstances:
A 2007 systematic review (1) identified 789 total EO cases reported worldwide since 1966. Most cases described years of HQ usage, not months. 95% (756/789) were from Africa where the nature of the HQ was unknown (there’s a huge black market in skin ‘bleaching’ creams that can contain just about anything, including very high doses of HQ combined with other EO-causing ingredients like phenol and resorcinol). EO can also result from the use of antimalarial drugs, widely used in Africa. Of the 137 cases that reported the HQ %, the majority (116) were 1-2%, but usage was unsupervised and excessive, with multiple daily applications⠀
22 of these cases were from the USA and involved the use of 1-2% hydroquinone used without supervision for years. These cases were reported from 1983-2006, which equates to 1 case per year, during which time an estimated 10-15 million HQ-containing creams/year were sold⠀
In an analysis of 20,814 patients who were prescribed 2-5% topical HQ for 8 weeks – 2 years, and closely medically supervised throughout this time, there were ZERO reports of EO⠀
So if you are using medically prescribed, <5% HQ, for a short duration and closely monitored, you have little to worry about on the EO front👌🏼⠀
And finally – does it cause cancer?
This is the most widespread myth on the internet about hydroquinone. But there isn’t a single published case of cancer in humans caused by topical application of HQ. This myth presumably comes from its association with tumours when it was FED to rats.
FOR MORE INFORMATION ABOUT WHETHER HYDROQUINONE IS RIGHT FOR YOU, CLICK HERE TO BOOK A VIDEO CONSULT WITH OUR EXPERIENCED MEDICAL TEAM.
Want to get started? Click here >
REFERENCES
1. Levitt J. The safety of hydroquinone: a dermatologist’s response to the 2006 Federal Register. J Am Acad Dermatol. 2007 Nov;57(5):854-72. doi: 10.1016/j.jaad.2007.02.020. PMID: 17467115.⠀
2. Tse TW. Hydroquinone for skin lightening: safety profile, duration of use and when should we stop? J Dermatolog Treat. 2010 Sep;21(5):272-5. doi: 10.3109/09546630903341945. PMID: 20095963.⠀
3. Searle T, Al-Niaimi F, Ali FR. Hydroquinone: myths and reality. Clin Exp Dermatol. 2020 Nov 7. doi: 10.1111/ced.14480. Epub ahead of print. PMID: 33159818.